Cascade Refrigeration Cycle Example¶
Imports¶
[1]:
from thermostate import State, Q_, units, EnglishEngineering as EE
from thermostate.plotting import IdealGas, VaporDome
Definitions¶
[2]:
sub_hiT = "R22"
sub_loT = "R134A"
T_1 = Q_(-30.0, "degF")
x_1 = Q_(1.0, "dimensionless")
p_2 = Q_(50.0, "psi")
p_3 = p_2
x_3 = Q_(0.0, "dimensionless")
delta_T_5 = Q_(-5.0, "delta_degF")
x_5 = Q_(1.0, "dimensionless")
p_6 = Q_(250.0, "psi")
p_7 = p_6
x_7 = Q_(0.0, "dimensionless")
Qdot_in = Q_(20.0, "refrigeration_tons")
Problem Statement¶
A two-stage cascade vapor-compression refrigeration system operates with R22 as the working fluid in the high-temperature cycle and R134A in the low-temperature cycle. For the R134A cycle, the working fluid enters the compressor as a saturated vapor at -30.0 fahrenheit and is compressed isentropically to 50 lbf/in.2 Saturated liquid leaves the intermediate heat exchanger at 50 lbf/in.2 and enters the expansion valve. For the R22 cycle, the working fluid enters the compressor as saturated vapor at a temperature 5 fahrenheit below that of the condensing temperature of the R134A in the intermediate heat exchanger. The R22 is compressed isentropically to 250 lbf/in.2 Saturated liquid then enters the expansion valve at 250 lbf/in.2. The refrigerating capacity of the cascade system is 20 ton_of_refrigeration. Determine
the power input to each compressor, in BTU/min
the overall coefficient of performance of the cascade cycle
Solution¶
1. the power input to each compressor¶
The power input to each compressor can be found by
To find the enthalpies, we need to fix all of the states.
The cascade refrigeration cycle is consistes of two smaller cycles both with 4 processes:
R22 cycle:
1. Isentropic compression
2. Isobaric heat exchange
3. Isoenthalpic expansion
4. Isobaric heat exchange
R134A cycle:
1. Isentropic compression
2. Isobaric heat exchange
3. Isoenthalpic expansion
4. Isobaric heat exchange
The following properties are used to fix the four states:
State |
Property 1 |
Property 2 |
|---|---|---|
1 |
\[x_1\]
|
\[T_1\]
|
2 |
\[p_2\]
|
\[s_2=s_1\]
|
3 |
\[p_3=p_2\]
|
\[x_3\]
|
4 |
\[p_4=p_1\]
|
\[h_4=h_3\]
|
5 |
\[T_5=T_3 + \Delta T_5\]
|
\[x_5\]
|
6 |
\[p_6\]
|
\[s_6=s_5\]
|
7 |
\[p_7=p_6\]
|
\[x_7\]
|
8 |
\[p_8=p_5\]
|
\[h_8=h_7\]
|
[3]:
st_1 = State(sub_loT, x=x_1, T=T_1)
h_1 = st_1.h.to(EE.h)
s_1 = st_1.s.to(EE.s)
p_1 = st_1.p.to(EE.p)
s_2 = s_1
st_2 = State(sub_loT, p=p_2, s=s_1)
h_2 = st_2.h.to(EE.h)
T_2 = st_2.T.to(EE.T)
st_3 = State(sub_loT, p=p_3, x=x_3)
T_3 = st_3.T.to(EE.T)
h_3 = st_3.h.to(EE.h)
s_3 = st_3.s.to(EE.s)
h_4 = h_3
p_4 = p_1
st_4 = State(sub_loT, p=p_4, h=h_4)
T_4 = st_4.T.to(EE.T)
s_4 = st_4.s.to(EE.s)
x_4 = st_4.x
T_5 = T_3 + delta_T_5
st_5 = State(sub_hiT, T=T_5, x=x_5)
h_5 = st_5.h.to(EE.h)
p_5 = st_5.p.to(EE.p)
s_5 = st_5.s.to(EE.s)
s_6 = s_5
st_6 = State(sub_hiT, s=s_6, p=p_6)
h_6 = st_6.h.to(EE.h)
T_6 = st_6.T.to(EE.T)
st_7 = State(sub_hiT, p=p_7, x=x_7)
T_7 = st_7.T.to(EE.T)
h_7 = st_7.h.to(EE.h)
s_7 = st_7.s.to(EE.s)
h_8 = h_7
p_8 = p_5
st_8 = State(sub_hiT, p=p_8, h=h_8)
T_8 = st_8.T.to(EE.T)
s_8 = st_8.s.to(EE.s)
x_8 = st_8.x
Plotting the T-s diagrams of both cycles,
[4]:
R22_vapordome = VaporDome(sub_hiT, ("s", "T"))
R134A_vapordome = VaporDome(sub_loT, ("s", "T"))
R22_vapordome.add_process(st_1, st_2, "isentropic")
R22_vapordome.add_process(st_2, st_3, "isobaric")
R22_vapordome.add_process(st_3, st_4, "isoenthalpic")
R22_vapordome.add_process(st_4, st_1, "isobaric")
R134A_vapordome.add_process(st_5, st_6, "isentropic")
R134A_vapordome.add_process(st_6, st_7, "isobaric")
R134A_vapordome.add_process(st_7, st_8, "isoenthalpic")
R134A_vapordome.add_process(st_8, st_5, "isobaric")
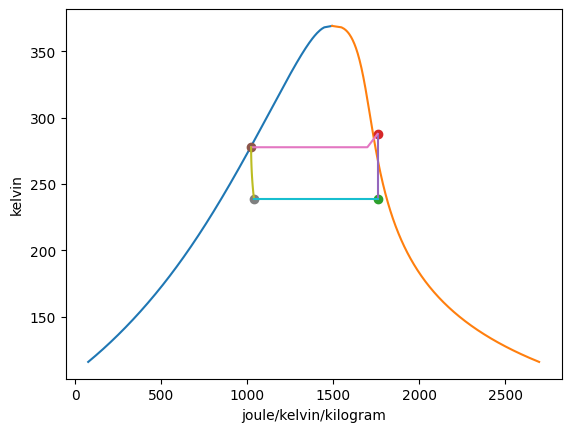
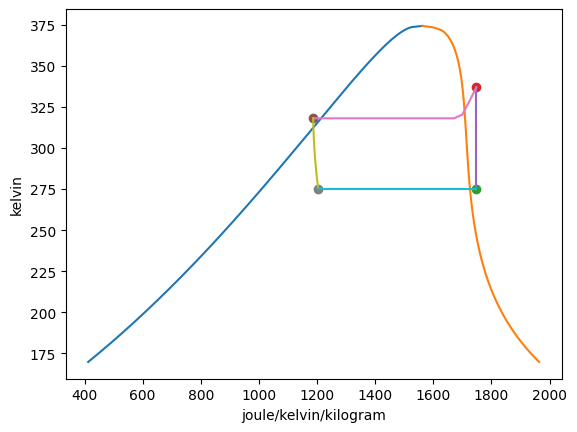
Summarizing the states:
State |
T |
p |
h |
s |
x |
phase |
|---|---|---|---|---|---|---|
1 |
-30.00 degF |
9.86 pound_force_per_square_inch |
162.30 btu / pound |
0.4196 btu / degR / pound |
100.00% |
twophase |
2 |
58.23 degF |
50.00 pound_force_per_square_inch |
176.42 btu / pound |
0.4196 btu / degR / pound |
— |
gas |
3 |
40.27 degF |
50.00 pound_force_per_square_inch |
88.65 btu / pound |
0.2442 btu / degR / pound |
0.00% |
twophase |
4 |
-30.00 degF |
9.86 pound_force_per_square_inch |
88.65 btu / pound |
0.2482 btu / degR / pound |
22.96% |
twophase |
5 |
35.27 degF |
76.59 pound_force_per_square_inch |
174.42 btu / pound |
0.4175 btu / degR / pound |
100.00% |
twophase |
6 |
146.75 degF |
250.00 pound_force_per_square_inch |
187.12 btu / pound |
0.4175 btu / degR / pound |
— |
gas |
7 |
112.76 degF |
250.00 pound_force_per_square_inch |
110.14 btu / pound |
0.2834 btu / degR / pound |
0.00% |
twophase |
8 |
35.27 degF |
76.59 pound_force_per_square_inch |
110.14 btu / pound |
0.2876 btu / degR / pound |
26.55% |
twophase |
The mass flow rate of the R134a is found from the refrigeration capacity
while the mass flow rate of the R22 is found from the intermediate heat exchanger
[5]:
mdot_r134a = (Qdot_in / (h_1 - h_4)).to("lb/min")
Wdot_loT = (mdot_r134a * (h_1 - h_2)).to("BTU/min")
mdot_r22 = mdot_r134a * (h_2 - h_3) / (h_5 - h_8)
Wdot_hiT = (mdot_r22 * (h_5 - h_6)).to("BTU/min")
Answer: The compressor work for the low-temperature cycle is \(\dot{W}_{\text{R134A}} =\) -766.52 btu / minute and the work for the high temperature cycle is \(\dot{W}_{\text{R22}} =\) -941.38 btu / minute
2. The coefficient of performance¶
The coefficient of performance of this refrigeration cycle is defined as
[6]:
beta = (Qdot_in / abs(Wdot_loT + Wdot_hiT)).to("dimensionless")
beta_max = (T_4 / (T_7 - T_4)).to("dimensionless")
Answer: The coefficient of performance is \(\beta =\) 2.34, while the maximum coefficient of performance for a refrigeration cycle operating between \(T_C =\) -30.00 degF and \(T_H =\) 112.76 degF is \(\beta_{\text{max}} =\) 3.01 dimensionless. The actual cycle has a lower COP, so it is possible.
